The Chemistry of Carbon Capture
Written by: Dorrie Peters
In a world where climate change is one of the most pressing issues, scientists are constantly coming up with fresh solutions on how to save and preserve the planet. Some ideas, such as carbon capture, have been pulled from history. Carbon Capture and Storage, or CCS, is a technique of separating carbon dioxide from other gasses first implemented in the 1920s. Given that carbon dioxide emissions are one of the leading contributors to global warming, CCS has the potential to make an invaluable impact. In fact, successful CCS technology can trap around 90% of CO2 from its emission source. However, the technology’s materials and risks make it difficult to execute. Noticing this, scientists have come up with new chemical solvents that could change CCS technology—and the climate crisis—as we know it.
How Does it Work?
Carbon capture has three main forms: post-combustion, pre-combustion, and oxyfuel combustion. All three methods work towards the same goal—to separate carbon dioxide from other gases—but each occurs at a different stage. Post-combustion is the most common and practical, as it can be built into factories and other sources of CO2 emissions. As the name implies, post-combustion CCS occurs after the burning of fossil fuels.
When burned for energy, fossil fuels create “flue gas”, which is a combination of carbon dioxide, water vapor, nitrogen, sulfur dioxide, and dirt particles. Flue gases are extremely harmful to both human bodies and the environment and are one of the most common forms of air pollution.
Figure 1
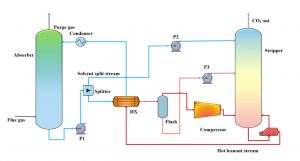
Model of the carbon absorption columns
Source: Sustainability of Carbon Capture and Utilisation
Post-combustion CCS technology gathers the flue gas before it has a chance to enter the atmosphere. Then, to accomplish the actual separation of CO2 from other gasses, a chemical solvent is employed. Certain chemical solvents have properties that absorb carbon dioxide while leaving other gasses untouched. The flue gas is exposed to these solvents in “absorption columns,” resulting in a CO2-rich liquid and a more environmentally clean gas. Finally, the gas is released and the CO2 is transported via pipes into storage facilities underground.
Flaws
One outstanding flaw with the idea of carbon capture is the element of storage. Because there is no safe way to get rid of carbon dioxide completely, carbon capture technology resorts to storing the gas in holding areas far underground via a process called geological sequestration. While this does prevent CO2 from entering the atmosphere and damaging the ozone layer, it has other harmful effects on the environment. Storing such large amounts of carbon dioxide in the earth is foreseen to alter the acidity of soil and deplete certain nutrients in water.
Another flaw in carbon capturing lies in the solvents used during the absorption step of carbon capture. In this step, chemicals are used to soak up the carbon and remove it from the rest of the gas. Historically, the most common solvents used have been anime solvents such as monoethanolamine (MEA) and diglycolamine (DGA). These two chemicals are abundant in the carbon capture world mainly because of their low cost and high reactivity. However, amine solvents require very high amounts of energy and absorb at a medium/low rate. In other words, they are often inefficient and would be unsuitable if CCS was a widespread practice.
New discoveries
Because of the efficiency and efficacy flaws related to standard CCS solvents, professionals at the forefront of chemistry have created several alternatives. Among them are advanced amine solvents, amino acid salts, carbonate systems, aqueous ammonia, immiscible liquids, and ionic liquids. While these new solvents vary in price and availability, they are all working towards more efficient absorption.
Figure 2.

Scientist David Heldebrant tests new, more energy-efficient solvents
Source: Pacific Northwest National Laboratory
Pacific Northwest National Laboratory is one example of a science institution at the forefront of carbon capture technology. Starting in the early 2010s, chemists such as David Heldebrant from PNNL have investigated the use of “water-lean” solvents, which have less H2O and more organic material than typical amine-based solvents. Because solvents with fewer water molecules have fewer bonds to be broken, they require less thermal energy to absorb carbon dioxide and are a better alternative for carbon capture processes
Conclusion
Given its high success rates and proximity to the largest factor of global warming, carbon capture has revolutionary potential in aiding the climate crisis. However, its efficacy must be improved if it is to function properly on a global scale. Problems related to the outdated and ineffective solvents used during the absorption process are being solved as new chemicals get tested to replace traditional amine compounds.
References and Sources
Cherepovitsyn, A. (2020, November 12). Popularization of Carbon Capture and Storage Technology in Society: Principles and Methods. International Journal of Environmental Research and Public Health, 17(22). 10.3390/ijerph17228368
Mumford, K. A. (2015, June 22). Review of solvent based carbon-dioxide capture technologies. Frontiers of Chemical Science and Engineering, 9, 125-141. https://link.springer.com/article/10.1007/s11705-015-1514-6
Oko, E., Wang, M., & Joel, A. S. (2017, February 27). Current status and future development of solvent-based carbon capture. International Journal of Coal Science & Technology, 4, 5-14. https://link.springer.com/article/10.1007/s40789-017-0159-0
Pacific Northwest National Laboratory. (2017, June 20). Next-gen solvents capture carbon with half the energy. Phys Org. https://phys.org/news/2017-06-next-gen-solvents-capture-carbon-energy.html
Vega, F., Cano, M., Camino, S., Gallego Fernández, L. M., Portillo, E., & Navarrete, B. (2018, August 16). Solvents for Carbon Dioxide Capture. Carbon Dioxide Capture, Chemistry, and Oil Recovery. 10.5772/intechopen.71443
1 thoughts on “The Chemistry of Carbon Capture”