Quantum Dots: a Nanoparticle that can Shape Our Future
In the field of nanotechnology, quantum dots, also called “semiconductor nanocrystals”, are semiconductor particles a few nanometres in size with optical and electronic properties that differ from those of larger particles via quantum mechanical effects. Quantum dots (QDs) are renowned for their ability to exhibit quantum size effects, special behaviors that tiny particles exhibit because they’re confined in small spaces, manifesting in intriguing optical and electronic properties.
What are they?
When a quantum dot is illuminated by UV light, an electron in the quantum dot can be excited to a state of higher energy. In the case of a semiconducting quantum dot, this process corresponds to the transition of an electron from the valence band to the conductance band. The excited electron can drop back into the valence band releasing its energy as light. This light emission (photoluminescence) is illustrated in Figure 1. The color of that light depends on the energy difference between the conductance and valence bands, or the transition between energy levels when the band structure becomes quantized due to the confinement of electrons within the small volume of the quantum dot.
Figure 1

Colloidal quantum dots are irradiated with a UV light. Differently sized quantum dots emit different colors of light due to quantum confinement.
Source: Boston University College of Engineering
Nanoscale semiconductor materials tightly confine either electrons or electron holes (lack of an electron at a position where one could exist). The confinement is similar to a three-dimensional particle in a box model. The quantum dot absorption and emission features correspond to transitions between discrete quantum mechanically allowed energy levels in the box that are reminiscent of atomic spectra. The atomic spectra is the spectrum of the electromagnetic radiation emitted or absorbed by an electron during transitions between different energy levels within an atom. For these reasons, quantum dots are sometimes referred to as artificial atoms, emphasizing their bound and discrete electronic states, like naturally occurring atoms or molecules. By coupling two or more such quantum dots, an artificial molecule can be made, exhibiting a mixing of their properties even at room temperature. Precise assembly of quantum dots can form superlattices that act as artificial solid-state materials exhibiting unique optical and electronic properties.
Quantum dots have properties intermediate between bulk semiconductors (part of the semiconductor far away from the middle of the two materials) and discrete atoms or molecules. Their optoelectronic properties change as a function of both size and shape. Larger QDs of 5–6 nm diameter emit longer wavelengths, with colors such as orange, or red. Smaller QDs (2–3 nm) emit shorter wavelengths, yielding colors like blue and green. However, the specific colors vary depending on the exact composition of the QD.
Applications
Quantum dots offer a vast array of applications across multiple industries, leveraging their unique properties for various technological advancements. Their high ability to absorb light and ultrafast optical nonlinearities (the response of a material to light is not directly proportional to the intensity of the light) make them particularly appealing for optical systems, showcasing potential in all-optical designs. With traits resembling single-electron transistors, they exhibit the Coulomb blockade effect, the decrease in electrical conductance at small bias voltages, hinting at their potential in quantum information processing as qubit implementations and as active elements in thermoelectrics. Moreover, their size tunability opens doors to diverse applications. Larger quantum dots tend to shift the spectrum towards the red, while smaller ones allow for subtle quantum effects to be harnessed.
In optics, quantum dots serve as diode lasers, amplifiers, and biological sensors, benefitting from their sharper density of states compared to structures with more dimensions. Quantum dots are also invaluable in optical encoding and combining two or more information channels into a single transmission medium, boasting broad excitation profiles and narrow, symmetric emission spectra. In biological analysis, they rapidly replace traditional organic dyes due to their superior brightness and stability. From single-particle tracking to highly sensitive cellular imaging, quantum dots revolutionize various biological imaging techniques.
Furthermore, quantum dots hold significant promise in photovoltaic devices, offering tunable absorption spectra and high ability to absorb light. They drive advancements in solar panel technology, potentially increasing efficiency and reducing costs. Quantum dot solar cells, including colloidal quantum dot photovoltaics, hybrid solar cells, and those integrated with nanowires, show potential for achieving higher photovoltaic efficiency. Additionally, quantum dots enhance light-emitting diodes (LEDs), improving color accuracy and energy efficiency. Quantum dot displays, utilizing blue-emitting LEDs and converting part of the emitted light into pure green and red light, deliver brighter screens with superior color ranges.
Moreover, quantum dots contribute to photodetector devices, photocatalysis, and antibacterial applications. They can be fabricated via solution-processing or conventional single-crystalline semiconductors, offering versatility for various photodetection applications. In photocatalysis, quantum dots serve as efficient photocatalysts for light-driven chemical conversions, including water splitting for solar fuel production.
Figure 2
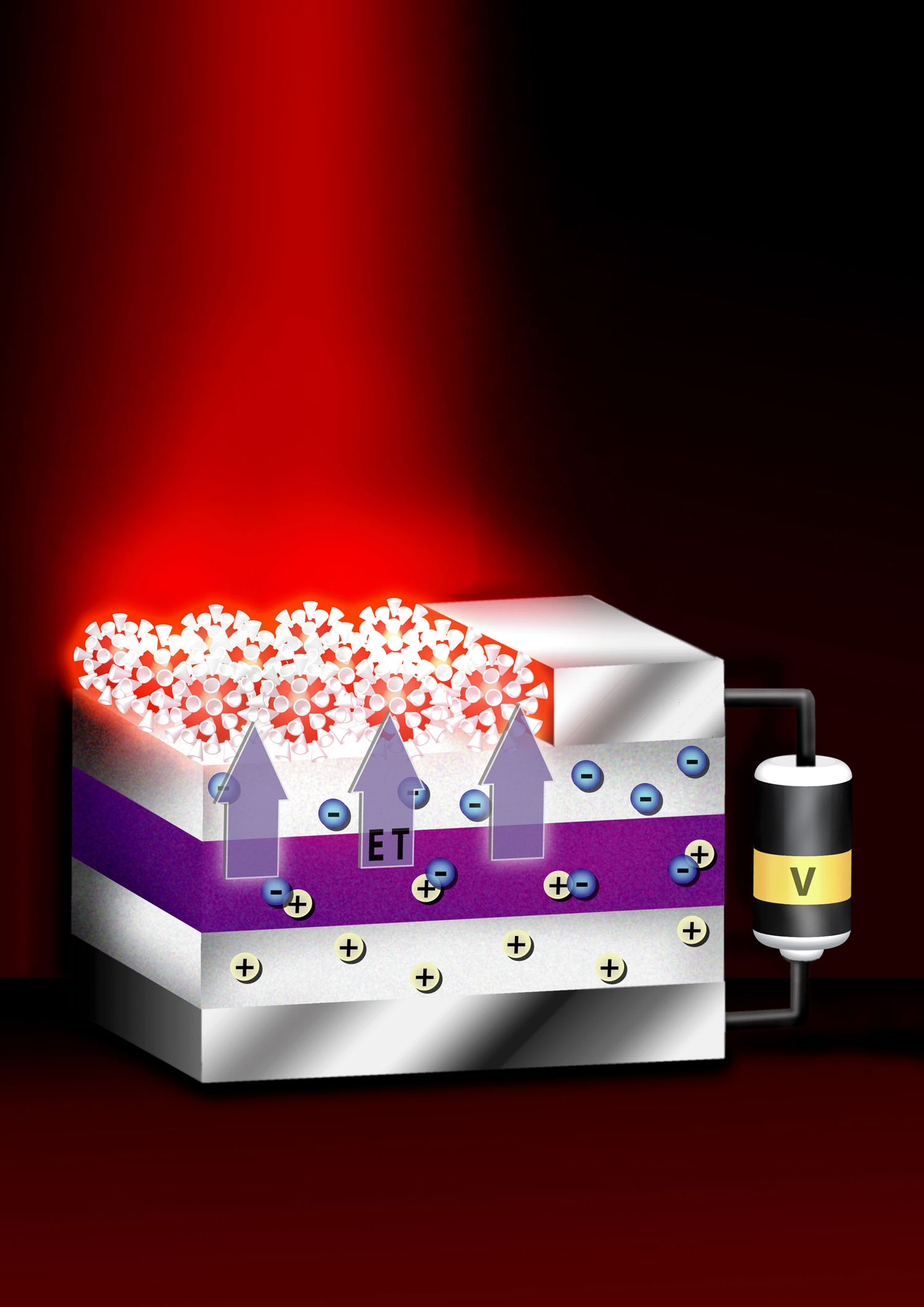
A device that produces visible light, through energy transfer from thin layers of quantum wells to crystals above the layers.
Source: Achermann, M.; Petruska, M. A.; Smith, D. L.; Koleske, D. D.; Klimov, V. I. (2004). “Energy-transfer pumping of semiconductor nanocrystals using an epitaxial quantum well”.
Conclusion
The world of quantum dots is a fascinating realm where nanoscale wonders and technological advancements converge. From their intricate quantum mechanical properties to their vast applications across industries, quantum dots continue to push the boundaries of science and innovation. As researchers look deeper into their synthesis, fabrication, and potential applications, quantum dots hold the promise of revolutionizing various fields, from electronics to medicine, paving the way for a future enriched with nanoscale marvels yet to be discovered.
References and Sources
Achermann, M.; Petruska, M. A.; Smith, D. L.; Koleske, D. D.; Klimov, V. I. (2004). “Energy-transfer pumping of semiconductor nanocrystals using an epitaxial quantum well”. Nature. 429 (6992): 642–646. Bibcode:2004Natur.429..642A. doi:10.1038/nature02571
Kastner, M. A. (1993). Artificial Atoms. Physics Today, 46(1), 24–31. https://doi.org/10.1063/1.881393
Moran, B. (n.d.). What Are Quantum Dots? | College of Engineering. Www.bu.edu. https://www.bu.edu/eng/2017/06/13/what-are-quantum-dots/
NMR Chemical Shifts of Impurities Charts. (2024). Merck, 1(1). https://www.sigmaaldrich.com/MX/en/technical-documents/technical-article/genomics/cloning-and-expression/blue-white-screening
Quantum Dot – an overview | ScienceDirect Topics. (n.d.). Www.sciencedirect.com. https://www.sciencedirect.com/topics/materials-science/quantum-dot